In June of 2017, Health Canada hosted a second round of cross-Canada industry consultations focused on their proposed Self-care Framework. These consultations were a continuation of the previous round of consultation sessions held in April and May of this year. Members of Source’s Regulatory Affairs Team attended both online and in-person consultation sessions to learn more about the progress of this important regulatory initiative.

Health Canada’s self-care initiative has the potential to change both pre- and post-market regulatory processes for Natural Health Products (NHPs), cosmetics and non-prescription drugs. Collectively referred to as self-care products, these products are intended for use without the direct oversight of a health care practitioner.
Although most self-care products are generally low-risk, Health Canada has differing compliance and enforcement approaches to each of these product categories.
NHPs, cosmetics and non-prescription drugs also each have their own regulatory requirements, although their respective product claims may be of a similar nature.
In the most recent version of the framework (June, 2017), Health Canada’s regulatory classification for self-care products would be based on the potential risk(s) of the product, whereby risk implies both product safety as well as potential harm of failed efficacy.
Self-care products would be classified into two main categories (Category I and Category II):
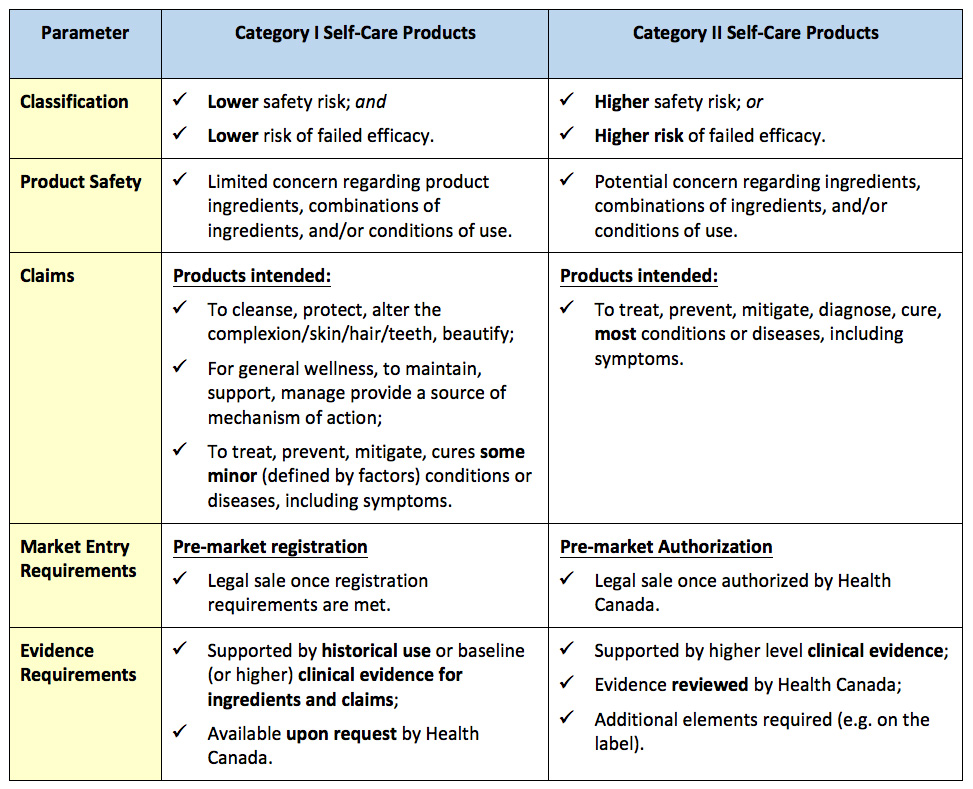
Stay tuned for further updates on this important regulatory modernization initiative, and feel free to connect with Source to discuss potential implications to your Canadian health products!




