On June 27, 2023, the Government of Canada announced the banning of cruel and unnecessary testing of cosmetic products on animals in Canada.
This release from Health Canada was intended to represent a major step forward in supporting animal welfare by reducing the Canadian market’s reliance on animal testing, while balancing the priorities of health and safety in product development research.
With this new legislation, companies in Canada will no longer be allowed to test cosmetic products on animals or use animal testing data to establish the safety of their cosmetics.
How does the Canadian public feel about ethical cosmetic testing overall?
The enactment of Bill C-47 marks a significant milestone for Canada, as it now prohibits both the inhumane and needless experimentation of cosmetic products on animals and prohibits the sale of items that have undergone such testing. There are exceptions, depending on the ingredients, there are still some mixed-use ingredients that will require animal testing (e.g., cosmetics containing pharmaceuticals).
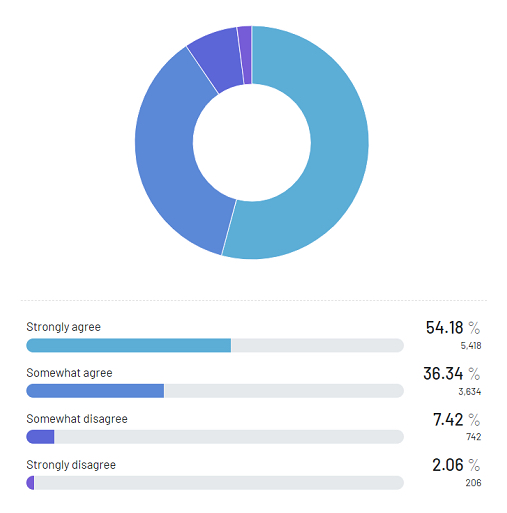
This holistic strategy towards eradicating animal testing within the cosmetics sector was chosen based on widespread support. According to a study by Real Research: over 54% of participants express strong agreement and over 36% indicate partial agreement with the prohibition on the sale of these products.
[Figure 1: Left, Percentage of respondents asked if they agree with Canada banning animal testing - courtesy of Real Research Media]
Why do I still see products tested on animals on shelves?
When browsing through the hair care or makeup aisles of your local drugstore, many of those products may still contain ingredients tested on animals, even after Canada's ban on cosmetic testing on animals took effect in December 2023.
This law does not apply retroactively. Instead, it focuses on prohibiting new animal testing within Canada and the sale of products relying on data and research derived from these types of studies. Additionally, amendments to the Food and Drugs Act, passed on June 22, also criminalize false claims of cruelty-free products by companies.
Health Canada is developing industry guidance and will enforce compliance based on trade or consumer complaints.
I’m bringing my cosmetic into Canada, how can I make sure my product will comply?
Even though most people consider it significant, this attitude showcases a change in consumer preferences favoring brands that prioritize animal welfare and ethical testing methods. Avoiding animal testing and carrying verifying claims will benefit your cosmetic product in other countries as well! Canada's prohibition falls in line with the international trend toward ethical cosmetic experimentation, following the lead of nations such as the United Kingdom, European Union, Australia, and South Korea, all of which have already implemented bans on cosmetic animal testing.
In personal care, brand trust—and proof of the product benefit—is everything
If you’re unsure if your cosmetic product meets requirements to comply with Bill C-47, our clinical trial and regulatory affairs teams can assist you.
How can Clinical Trial Data help to Support Safety & Efficacy
Clinical trial data is often the missing piece between a compelling claim and the audience you’re trying to reach. Through a well-designed clinical trial, you’ll be able to validate your label claims and gain a competitive advantage in a flooded marketplace.

SNI's cosmetic safety and efficacy clinical capabilities include:
- Efficacy Testing: Skincare, Hair and Scalp Care - Moisturization and Hydration, Sunscreen Safety Testing.
- Safety and Sensitivity Testing
- Anti-aging: Firmness/Elasticity, Hyperpigmentation, Wrinkles/Fine Lines, Dark circles/puffiness, Cellulite.