In 2022, Health Canada introduced a new set of regulations for a newly defined subcategory of food products called Supplemented Foods. These are prepackaged foods that contain added supplemental ingredients.
Background: Before the implementation of the Supplemented Food Regulations, any food containing supplemental ingredients (SIs) such as caffeine, vitamins, or herbal extracts required a Temporary Market Authorization (TMA). To obtain this authorization, applicants had to submit a request to Health Canada, which would assess whether the product’s deviation from existing food regulations was acceptable. If sufficient evidence was provided, Health Canada would issue a Temporary Market Authorization Letter (TMAL) allowing the product to be sold in Canada for a limited period, usually five years.
Recognizing that this process was not a sustainable approach for regulating innovative products, Health Canada developed new regulations to enable faster market entry. These regulations were informed by data collected during TMA approvals and established a structured list of approved supplemental ingredients, permissible thresholds, and required cautionary statements for certain ingredients.
Due to the difference in use between conventional foods, which can be consumed without limits, and supplemented foods, which have consumption limits due to potential risks associated with high levels of supplemental ingredients, an additional set of labelling regulations was developed specifically for this new food category.
These product-specific labelling requirements help consumers make informed choices by providing clear and accurate information while distinguishing Supplemented Foods from conventional foods. Products that contain added nutrients or bioactive ingredients must adhere to strict regulatory guidelines to ensure transparency and compliance.
Learn more about the labelling requirements for Supplemented Foods in our post below.
What Are Supplemented Foods
Definition of Supplemented Foods

Supplemented Foods are prepackaged products that have intentionally added supplemental ingredients added for purposes other than nutrition. These ingredients include vitamins, minerals, amino acids, caffeine, and herbal extracts. Unlike conventional foods, supplemented foods contain ingredients that require specific conditions of use to ensure safe consumption. As such, a subset of regulations was developed to ensure proper regulatory oversight and consumer protection.
As defined in the FDR: “supplemented food means a prepackaged product that belongs to a food category set out in column 1 of the List of Permitted Supplemented Food Categories and to which a supplemental ingredient has been added, but does not include
- a food for special dietary use as defined in section B.24.001 and referred to in any of paragraphs B.24.003(1)(f) to (f.2) and (h) to (j), even if the food for special dietary use is also a gluten-free food referred to in paragraph B.24.003(1)(g);
- a food that is labelled or advertised for consumption by (i) infants as defined in section B.25.001, (ii) children one year of age or older but less than four years of age, or (iii) women who are pregnant or breastfeeding;
- any of the following foods set out in column I of the Table to section D.03.002: (i) a food referred to in any of items 1, 2.1, 2.2, 4, 5, 7, 8, 9.1, 10 to 13, 15, 17 to 19, 21 to 25 and 27, and (ii) prepackaged ice;
- a food that has not been processed or that has been minimally processed; or
- a beverage with an alcohol content of more than 0.5%; (aliment supplémenté).”
Differences Between Supplemented and Fortified Foods
Although both supplemented and fortified foods involve the intentional addition of nutrients, their purposes and regulatory frameworks differ significantly. Fortified foods are designed to prevent or correct nutrient deficiencies in the general population. A common example is milk fortified with vitamin D. In Canada, only certain foods can be fortified with vitamins and minerals, and their fortification must adhere to specific nutrient levels established by the Food and Drug Regulations (FDR).
In contrast, supplemented foods are formulated to meet specific health, dietary, or lifestyle needs. These foods may contain a variety of ingredients that offer targeted benefits, but they are not primarily intended to address nutrient deficiencies in the population. For example, an energy drink that contains caffeine serves to promote alertness and energy rather than to correct a widespread deficiency.
While fortification of certain foods is mandatory in Canada, meaning specific foods must be fortified in accordance with the FDR before entering the market, supplemented foods are not subject to such requirements. Manufacturers have the flexibility to voluntarily include approved ingredients in supplemented foods based on their product formulation, provided they stay within established ingredient-specific limits.
Importance of Supplemented Foods in Canada
In Canada, supplemented foods are used to help support varied dietary preferences and health goals. These products are in high demand among consumers looking for a variety of functional benefits including more energy, relaxation, or nutritional supplementation.
For instance, an on-the-go professional might benefit from a supplemented protein shake with supplemental magnesium for muscle recovery post-workout. Under increasing consumer demand, the supplemented food market has multiplied, making clarity in labelling, compliance, and faster route to market essential.
Overview of Supplemented Food Regulations
The primary goal of Canada’s Supplemented Food Regulationsis to protect public health while providing manufacturers with clear and straightforward standards and a defined pathway to market. These regulations emphasize the importance of distinguishing supplemented foods from conventional foods to ensure transparency for consumers and reduce the potential risk of adverse effects associated with certain supplemental ingredients.
A key aspect of these regulations is the establishment of the Supplemented Food Facts table (SFFt), and the use of the Supplemented Foods Caution Identifier (SFCI) and caution statements. These tools help differentiate supplemented foods from conventional foods, which instead use the standardized Nutrition Facts table (NFt).
These differences provide a clear, at-a-glance way for consumers to distinguish supplemented foods from conventional products in stores, helping to prevent accidental confusion and improper consumption.
Key Compliance Steps for Businesses
Transitioning from Temporary Market Authorizations to Supplemented Food Regulations
Businesses that previously operated under a Temporary Market Authorization for supplemented foods must now comply with the finalized Supplemented Food Regulations. These authorizations allowed the sale of specific products while Health Canada developed regulatory guidance and assessed supplemental ingredients.
Although permitted for use in foods during the Temporary Market Authorization period, some ingredients were not included in the final List of Permitted Supplemental Ingredients. As such, as the transition period to the Supplemented Foods framework takes place, manufacturers must update their formulas to align with the new regulations. For example, some caffeinated beverages were permitted to contain L-carnitine under the TMAL, but this ingredient is not permitted for use in supplemented foods. As a result, manufacturers must ensure that L-carnitine is removed from their Canadian products.

Transitioning from a TMAL to the new Supplemented Food Regulations requires companies to review their product lines and ensure compliance with the List of Permitted Supplemented Food Categories and the List of Permitted Supplemental Ingredients. Products that do not fall within an approved category cannot contain supplemental ingredients. Similarly, products in an approved category that include ingredients not listed as permitted must be reformulated to exclude those ingredients.
New Supplemented Foods Entering the Canadian Market
If you are a manufacturer of a product that previously did not apply for a TMAL, you can now sell supplemented foods under the new Supplemented Food Regulations. The key responsibility for manufacturers is to ensure that the foods being supplemented are allowed for supplementation, contain only the ingredients listed in the permitted supplemental ingredients list, and adhere to the specified levels and conditions of use. Additionally, manufacturers must ensure their product labelling meets the requirements outlined for this food category.
Compliance Deadline
Manufacturers with a valid TMAL at the time of the publication of the Supplemented Food Regulations(July 21, 2022) have until December 31, 2025 to comply with the new regulations.
All other products must comply with the regulations starting from the publication date.
Labelling Requirements for Supplemented Foods
In Canada, supplemented food labels must include specific information to comply with federal regulations. All the requirements under the Food and Drug Regulations that apply to conventional pre-packaged foods also apply to supplemented foods. However, there are key differentiators that manufacturers must consider when labelling supplemented foods:
Supplemented Food Facts table: This table provides essential information about the supplemental ingredients used in the product, ensuring that consumers are informed about the added nutrients or bioactive substances.
Supplemented Caution Identifier: This identifier is a clear marking that highlights any potential risks or special considerations related to the product, such as the presence of ingredients that may not be suitable for certain individuals or have specific usage limits.
Caution Statements: These statements are required when certain ingredients present a potential health risk, providing consumers with clear warnings about proper use and any associated concerns.
As discussed earlier, these labelling requirements are crucial for distinguishing supplemented foods from conventional foods.
Below is a high-level overview of some of the labelling requirements for supplemented foods. For additional information and full breakdown of the regulations, please reference Guidance document: Supplemented Foods Regulations.
1. Supplemented Food Facts table (SFFt)
All supplemented foods are required to display a standardized Supplemented Food Facts table (SFFt) that corresponds to the size of the principal display surface (PDS). Often referred to as a modified Nutrition Facts table (NFT), the SFFt closely resembles the traditional NFt but includes additional sections. The key addition is a “Supplemented with” section, which outlines the supplemental ingredients added to the product, their amounts, and, where applicable, their % Daily Value (DV).
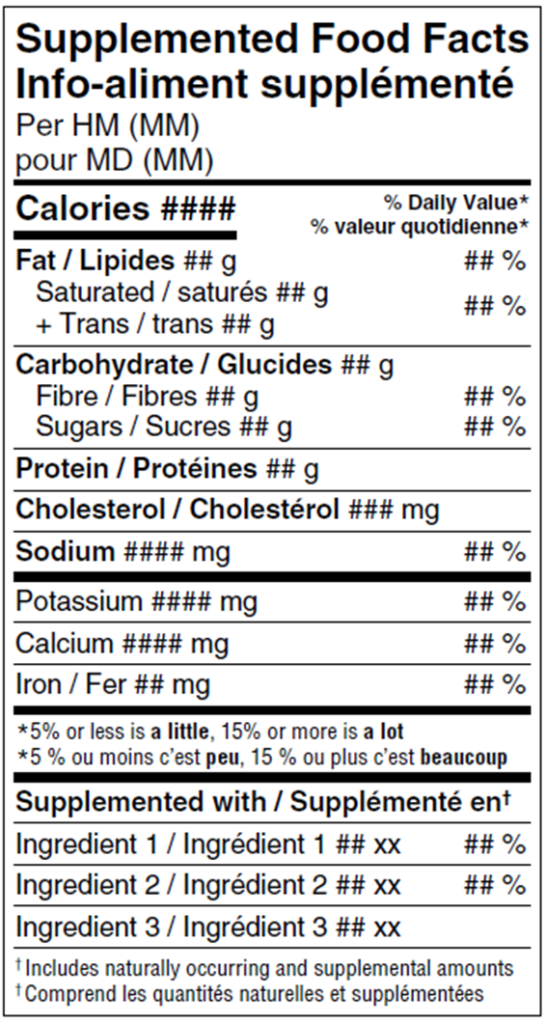
Instead of the “Nutrition Facts” title, the table for supplemented foods is labelled as the “Supplemented Food Facts” table. As with conventional foods, the SFFt must appear in both English and Canadian French to meet the regulatory requirements.
2. Supplemented Foods Caution Identifier (SFCI)
Some supplemented foods must carry a caution identifier displayed on the principal display panel (PDP) (i.e., panel seen at the time of purchase) when the supplemental ingredients in the product trigger an addition of a caution statement. In other words, not every supplemented food has to have this identifier, but it must be included if a supplemental ingredient with a prescribed risk statement is used in the product. To determine if a risk statement is required, manufacturers should reference the approved supplemental ingredients and their conditions of use.

When required, the SFCI must meet both bilingual and technical requirements. Similar to the SFFt, the SFCI is standardized and must appear in the same format on all supplemented foods sold in Canada. It should be placed in a clear, uncluttered area and be proportionate to the size of the principal display panel (PDP). Additionally, the SFCI must be displayed in both official languages or in a bilingual format.
Based on the product’s packaging orientation and size, the appearance, placement, buffer zone, and size might differ. For instance, for a supplemented food with a PDP that is taller than its width, the SFCI should be placed on the right half of the panel. For all other supplemented foods, the SFCI should be positioned on the upper half of the principal display panel. For additional information please refer to the Food and Drug Regulations.
The voluntary use of the SFCI, or any symbol resembling it, is prohibited. The SFCI should only be used when a caution statement triggered by the supplemental ingredients in the product, is present on the label.
3. Caution Statements
As mentioned in earlier sections, the use of certain supplemental ingredients requires a caution statement to be included on the supplemented food product. These caution statements are often linked to the type of ingredient or its quantity. The specific caution statement will vary depending on the supplemental ingredient added to the product.
For example, if biotin is added at 150 µg per serving to a carbonated beverage, the following caution statements would be required:
- Not recommended for those under 14 years old
- Do not drink on the same day as any other supplemented foods or supplements containing biotin
- Do not exceed 1 serving per day
When triggered, caution statements must be displayed in a standardized format, grouped under the “Caution:” title, and placed next to the Supplemented Food Facts table (SFFt). The caution statements should be enclosed in a border, and their formatting must align with the format used for the ingredient declaration and allergen/cross-contamination statements.

For additional requirements on manner of declaration and technical requirements, please reference the Food and Drug Regulations.
Bringing US Supplemented Foods to Canada
When importing supplemented foods into Canada, it is crucial to have a clear understanding of the country’s specific regulations to ensure that the product’s formula complies with ingredient-specific rules and food category requirements. This includes meeting the necessary safety, quality, and labelling standards prior to product’s import.

While there are no licensing requirements for foods like there are for Natural Health Products, food importers must ensure that the items being imported into Canada are properly declared on import forms in advance.
For manufacturers, it is equally important is ensuring that the Canadian importer holds the appropriate licensing for their activities. For example, Canadian importers must obtain a Safe Food for Canadians Licence for food importation and must follow the food-specific import requirements. This ensures that the importer is compliant with all regulations governing the importation, handling, and distribution of supplemented foods within Canada.
To Summarize

The introduction of Canada’s Supplemented Food Regulations has provided a structured and transparent framework for businesses looking to innovate within this evolving food category. By establishing clear ingredient thresholds, cautionary labelling, and compliance requirements, these regulations help ensure consumer safety while facilitating market entry for manufacturers. Properly understanding and adhering to these guidelines is essential for companies seeking to introduce new supplemented food products in Canada or transition from Temporary Market Authorizations.
Compliance with labelling and regulatory requirements is not just a legal necessity but also a means of building trust with consumers. By providing clear, standardized information on supplemented food products, businesses can empower consumers to make informed choices about their health and wellness. For importers, ensuring that all documentation, licensing, and regulatory approvals are in place is critical to avoiding delays and ensuring seamless market entry.
Navigating Canada’s Supplemented Food Regulations may seem complex, but with the right support, it can be a straightforward process. Whether you are formulating a new product, updating existing labels, or expanding into the Canadian market, SNI’s Regulatory Team is here to assist you.
Contact us for expert guidance and customized solutions tailored to your business needs. Let’s drive innovation and compliance forward together! Email us at info@sourcenutra.com to learn more or to request a quote.