- General Overview of Nutrition Facts Table Regulations
- Mandatory Nutrients Declared in Nutrition Facts Tables (NFts)
- Rounding Rules for Nutrition Values in the Nutrition Facts Table
- Review of Key Nutrition Facts Table (NFt) Elements
- Declaring Nutrient Information Outside of the Nutrition Facts Table
- Bilingual Requirements for Nutrition Facts Tables
- Understanding Nutrition Facts Table (NFt) Format Families
- 5 Steps to Choosing the Right Nutrition Facts Table (NFt)
- Final Remarks
- FAQ
- What information must appear in the Nutrition Facts table in Canada?
- How do I determine the correct serving size for the NFt?
- Which foods are exempt from showing a Nutrition Facts table?
- How do I choose the right NFt format or size for my product?
- Can I add nutrient or ingredient information outside the Nutrition Facts table?
As with any food product, nutritional information on prepackaged foods serves as a core component of the label, helping consumers make informed purchasing decisions, and supporting a more conscious approach to nutrition. For this reason, nutrition panels have become a standard labelling requirement in many jurisdictions worldwide. However, the method of declaration, nutrient listings, and visual presentation of this information differ significantly between countries.
The summary below highlights key standards that every food manufacturer or importer should review before entering the Canadian market to ensure full compliance.
Please note that this article provides a general overview and should not replace professional regulatory guidance. For questions about Nutrition Facts tables (NFts) or broader food labelling regulations, contact our team at info@sourcenutra.com for tailored support, service details, and pricing information.
General Overview of Nutrition Facts Table Regulations
In Canada, the requirements for nutritional information are distinct and must be followed closely to ensure compliance and meet consumer expectations. These rules differ from those of neighbouring markets, and misunderstanding these differences is a common barrier to market entry for international brands.
In Canada, the Nutrition Facts table (NFt) is one of the most important components of a food label. It sits within a larger regulatory system outlined in the Food and Drug Regulations (FDR) under Division B.01.401 to B.01.406, which sets out what must be declared and how it must appear. Health Canada determines the nutrient list and the Daily Values that form the basis of the NFt, while the Canadian Food Inspection Agency (CFIA) enforces the formatting, layout, and presentation requirements under the Food and Drugs Act and the Safe Food for Canadians Act. Together, these requirements outline precise expectations for font size, contrast, order of information, and the limited flexibilities permitted for small or unusually shaped packages.
Mandatory Nutrients Declared in Nutrition Facts Tables (NFts)
The standardized display of nutrient information on prepackaged foods plays a vital role in supporting consumer literacy and helping individuals understand the nutritional composition of the products they consume. These standardized tables are designed to make it easy for consumers to compare products, whether within the same or across different food categories. The ultimate goal is to empower consumers to make healthier purchasing decisions, contributing to Canada’s broader public health objective of reducing diet-related chronic diseases.

Before the introduction of the new Front-of-Package (FOP) Nutrition Symbol regulations, the NFt was the only tool that allowed consumers to identify key nutrients and determine whether a food contained high levels of nutrients of concern, such as saturated fat, sugars, or sodium.
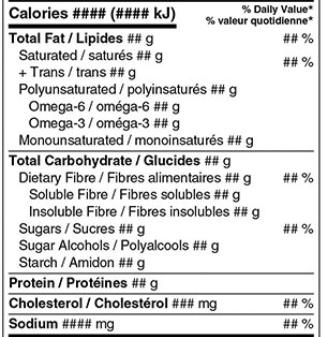
Under the FDR, most prepackaged food products must display an NFt that corresponds appropriately to the package’s size and layout. The NFt presents core nutrient information in a standardized format and includes only the data and nutrients permitted under the regulations.
The information that must appear in the NFt, both in English and in French, includes the following:
- “Nutrition Facts” title
- Serving size declaration
- Calories
- Fat (saturated and trans) (declared in g)
- Carbohydrates (fibre and sugars) (declared in g)
- Protein (declared in g)
- Cholesterol (declared in mg)
- Sodium (declared in mg)
- Potassium (declared in mg)
- Calcium (declared in mg)
- Iron (declared in mg)
- * 5% or less statement (declared at the bottom of the table)
- % Daily Value (declared in % based on the declared amount)
This information, for most foods, is presented in the following bilingual format:
Rounding Rules for Nutrition Values in the Nutrition Facts Table
In addition to ensuring that all core nutrient information is presented in the NFt, manufacturers should ensure that proper rounding rules are applied to the nutritional information and the correct nomenclature is followed.
For instance, the declaration of the serving size (i.e., serving of stated size) is standardized for the verbiage, units, and rounding rules that must be used:

Nomenclature for serving size declaration is limited to:
- Serving Size (naming the serving size)
- Serving (naming the serving size)
- Per (naming the serving size)
Units that can be used are also limited to the following:
For single-serving packaged products, the serving size must be declared:
- Per package, and;
- In grams of milliliters
- For example: “Per 1 bottle (355 mL)”
For multiple-serving pre-packaged products:
- The household measure (HM) (which must also correspond to the Table of Reference Amounts), and;
- The metric measure (MM) that applies to the product (which must also correspond to the Table of Reference Amounts)
- For example: “Per ½ cup (85 g)”
Rounding of serving size follows strict thresholds. :
- Amounts below 10 grams or 10 millilitres must be rounded to the nearest 0.1 g or 0.1 mL.
- Amounts of 10 grams or 10 millilitres or more must be rounded to the nearest whole number.
- Fractions must be written with a numerator and denominator separated by a line, such as ½ cup.
- Composite declarations for assorted foods must include the word “assorted,” for example, Per 5 assorted candies (15 g).
Review of Key Nutrition Facts Table (NFt) Elements
The Serving Size Requirements
All nutrition information in the NFt is based on the edible portion of the food. The serving size must be declared directly beneath the Nutrition Facts heading using an approved manner of declaration. It reflects the quantity typically consumed at a single eating occasion and is generally based on the food as sold.

Certain categories, however, follow a different approach. Coffee and tea serve as key examples where the serving size is based on the food “as consumed,” since the product is not eaten or drunk in its sold form. Foods that require preparation or are usually combined with other ingredients, such as pudding mix or cereal served with milk, must declare serving size for the food as sold and may optionally declare the serving size as prepared.
Serving sizes are designed to align with the regulated reference amounts (RAs), which reflect the quantities typically consumed in one sitting. This consistency allows consumers to more easily compare similar products when making purchasing decisions. To maintain standardized presentation, the serving size must be declared in the NFt by listing the household measure (HM) first, followed by the corresponding metric measure (MM) in brackets and following the rounding rules outlined under the regulations.
Serving Size for Single-Serving and Multi-Serving Foods
Lastly, additional considerations apply when determining serving sizes for single serving and multiple serving prepackaged foods. These two categories are treated differently under the nutrition labelling provisions in the FDR, and the distinction must be applied correctly to ensure the NFt is compliant.
A single-serving prepackaged product is defined as a food that either
- has a net quantity less than 200 percent of the applicable reference amount, or
- would reasonably be consumed by one person at a single eating occasion.

When a product meets this definition, the serving size in the NFt must represent the entire contents of the package. This ensures that the nutrient values reflect the full amount the consumer is expected to eat at once. Common examples include individual beverages up to 473 mL, single granola bars, small ready to drink smoothies, or single portion dips.

A multi-serving prepackaged product contains more food than is typically consumed at one eating occasion and does not qualify as a single-serving under the criteria above. For these products, the serving size declared in the NFt must be based on the regulated reference amount, using the household measure and corresponding metric measure specified in Columns 3A and 3B of the Table of Reference Amounts. The serving size should not default to the entire package unless the whole amount would reasonably be eaten in one sitting, which is uncommon for larger or family sized products.
Accurately distinguishing between single serving and multiple serving prepackaged foods ensures that Nutrition Facts tables are standardized, comparable across product categories, and fully aligned with the technical requirements of the regulations. This distinction also supports consumer understanding by presenting nutrition information that reflects realistic consumption patterns and regulatory intent.
The Role of Reference Amounts (RAs)
RAs form the technical foundation of serving size determination. These regulated quantities represent the amount of food typically consumed at a single eating occasion and apply to both single-serving and multiple-serving formats. Established by Health Canada and incorporated by reference into the FDR, RAs promote consistency across categories and support accurate nutrient content claims and health claims.
RAs reflect the food in its ready-to-serve form unless otherwise noted. Only the edible portion is considered, and non-edible components or liquids that are not customarily consumed are excluded. The Table of Reference Amounts for Food covers 24 food categories and provides detailed guidance on calculating and declaring serving sizes for complex or multi-portion products. These standardized reference points ensure that NFt information remains aligned across the market, reducing variability and supporting consumer understanding.
Daily Value (DV) and Percent Daily Value (%DV)
The Daily Value (DV) acts as the nutritional benchmark for the percent Daily Value (%DV) that appears in the NFt. It reflects the recommended daily intake for key nutrients and serves as the foundation for how nutrient content and health claims are assessed. Established by Health Canada and outlined in the Table of Daily Values, these amounts are tailored to specific age groups and nutrient categories to ensure consistency and accuracy across food products.
The %DV communicates how much of a particular nutrient is found in one serving compared to the total amount recommended for a day. This helps consumers quickly understand whether a food contains a little or a lot of a certain nutrient. The calculation, expressed as a simple percentage, is governed by specific rounding and declaration rules that manufacturers must follow to ensure accuracy and compliance. Ultimately, the %DV allows consumers to compare foods easily and make informed dietary choices aligned with Canada’s healthy eating recommendations.
Additional Nutritional Information
The NFt also extends beyond the declaration of core nutrients and can include additional information depending on the product and its composition. Some foods are permitted to display extra nutrient details on a voluntary basis, while others are required to do so under specific regulatory conditions.
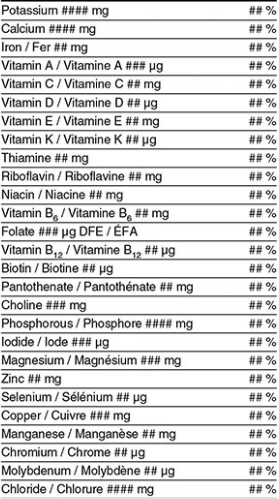
For example, certain vitamins, minerals, or fatty acids may be added to the NFt when a manufacturer chooses to highlight them on the package or in product marketing. When such nutrients are declared, their presentation must strictly follow the FDR, including the prescribed order, rounding conventions, and calculation of the %DV based on the Table of Daily Values. This ensures that voluntary declarations maintain the same level of accuracy, consistency, and comparability as mandatory ones.
In other cases, additional nutrient information must be included in the NFt when triggered. This occurs when the nutrient in question is referenced elsewhere on the packaging or in advertising, which triggers mandatory disclosure within the NFt to maintain consistency and prevent misleading representation. One example is when a product label or advertisement highlights the amounts of omega-3, omega-6, or monounsaturated fatty acids. When referenced elsewhere on the label or in advertisement, these nutrients must also be declared within the NFt. The same principle applies to any other nutrient that is voluntarily mentioned outside of the NFt, such as fibre, specific vitamins, or minerals. This requirement ensures that consumers receive complete and accurate information directly from the standardized table, allowing them to compare products objectively and make well-informed nutritional choices.
Declaring Nutrient Information Outside of the Nutrition Facts Table
Quantitative declarations of nutrients, energy value, or supplemental ingredients may appear outside of the NFt when permitted. These statements can be placed elsewhere on the label or in advertisements, provided they are accurate, expressed per serving of stated size, and comply with prescribed units and formats.

When a quantitative declaration is made, it must use the correct measurement units (such as grams, milligrams, or micrograms) and may also include a %DV where applicable. However, such statements cannot be qualified with words that could mislead consumers, such as “only” or “less than.”
Making a quantitative statement can also trigger additional disclosure requirements. For example, referencing a specific fatty acid may require declaring related fatty acid groups within the NFt. Foods that are typically exempt from carrying a NFt may lose that exemption if a quantitative statement is added to the label, except for products that are always exempt.
Overall, quantitative declarations outside the NFt are permitted but strictly regulated.
Bilingual Requirements for Nutrition Facts Tables
As with all other mandatory food label elements, the nutrition labelling must appear in both English and French to comply with Canada’s bilingual labelling requirements. Most manufacturers choose to use a bilingual NFt that combines both languages within a single panel. This bilingual standard format is preferred because it conserves space while maintaining full compliance with the FDR. When using a bilingual format, manufacturers may present the English text first followed by the French equivalent, or display the French text first followed by the English equivalent.

It is also acceptable to display two separate unilingual NFts, one in English and one in French. In this case, each table must meet the applicable size and legibility requirements according to the available display surface. When two NFts are used, they must appear in equally prominent locations and with the same visual emphasis to ensure that both official languages are presented clearly and fairly. This format is sometimes used on packaging with two distinct sides, where one panel is entirely in French and the other in English. However, most manufacturers prefer a single bilingual NFt, as it conserves space and simplifies label design, while still maintaining regulatory compliance.

Understanding Nutrition Facts Table (NFt) Format Families
In addition to ensuring that all required information is declared in the NFts, manufacturers must also ensure that they are using correct NFt formats. In some cases, a few flexibilities are permitted, allowing manufacturers to choose between the NFt formats that suit their products best. In other cases, NFt formats available for specific products are limited. When selecting the appropriate NFt format, manufacturers must first determine the available display surface (ADS) of their package.
Available Display Surface and Nutrition Facts Table Format
The ADS refers to the total area of a prepackaged food container where a label can be applied and displayed. For most products, this includes the entire surface area of the package, such as the sides and bottom, provided the product would not be damaged or leak if inverted.

Areas excluded from ADS calculations include the UPC, any portion destroyed upon opening (excluding single-serving packages), the bottom of packages that would be damaged or spill when turned upside down, any surface where a label cannot be applied, and areas where information cannot be displayed or read clearly. Additional exceptions apply to ornamental containers and product tags, which are not covered in this overview.
Once the ADS has been calculated, manufacturers must use this measurement to determine the corresponding 15% area of the package. According to CFIA guidance and the FDR, the NFt is generally not required to occupy more than 15% of the ADS for most prepackaged foods. However, the selected NFt format and size should be proportionate to the available space and reasonably close to that 15% threshold to ensure legibility and prominence. If 15% of the ADS exceeds the dimensions of the largest prescribed NFt format, manufacturers are not required to enlarge the table beyond the maximum size permitted under the regulations. In such cases, using the largest compliant NFt format remains acceptable, even if it occupies less than 15% of the ADS.
How to Calculate Available Display Surface
For example, a manufacturer is designing a label for a cylindrical container of soup with an available display surface (ADS) of 260 cm². To determine the 15% area, the manufacturer calculates: 15% of 260 cm² = 39 cm²

According to CFIA guidance, the NFt does not need to exceed this 39 cm² area, but the chosen format should be as close to that value as practical while maintaining readability and regulatory compliance. When reviewing the Directory of Nutrition Facts Table Formats, the manufacturer finds that the bilingual standard format NFt occupies approximately 35.38 cm², which fits proportionally within the available space and remains legible. Since the next largest format would exceed the panel space available, the current format is acceptable, even though it occupies less than 15% of the ADS. In this example, the manufacturer meets the regulatory requirements by using the largest compliant NFt that fits within the product’s design, without being obligated to expand the table beyond the prescribed dimensions.
In addition to choosing different format based on size, manufacturers can choose between format “families”, which allow companies to present the NFt information in manner that’s best suited for their packaging.
Understanding Format Families for Nutrition Facts Tables
Once the ADS has been determined, the next step is to select the most appropriate NFt format family. The FDR outlines several families of formats, each designed for a specific type of food product or presentation scenario. These format families ensure that nutrition information is displayed in a clear, standardized manner suitable for the product’s composition, packaging, and intended use. When choosing a format family, manufacturers must also consider how the food is typically prepared or consumed, whether it is sold as a single-serving or multiple-serving product, and whether it contains more than one component that requires separate nutrient declarations.
Standard, Simplified, and Dual Nutrition Facts Table (NFt) Formats
The standard, horizontal, or linear format family is the most common and is typically used for foods sold in a single form, such as milk, bread, or other ready-to-eat products. Other format families apply to more specialized products. For example, the simplified formats are designed for foods that contain very low amounts of nutrients where six or more of the core nutrients and calories are reported as zero, while dual format for foods requiring preparation is used for products sold unprepared but typically consumed after cooking or mixing, such as powdered drink mixes or dry soups.

Aggregate Nutrition Facts Table (NFt) Formats
Products that contain multiple food types or components may require an aggregate format, which lists separate nutrient information for each part of the product, such as a snack kit containing crackers and cheese. Age-specific products, such as foods intended for infants, also have dedicated format families with their own exceptions.

Selecting the correct NFt format family ensures that the label accurately represents how the food is consumed and helps Canadian consumers easily interpret the nutrition information, supporting both regulatory compliance and transparency.
5 Steps to Choosing the Right Nutrition Facts Table (NFt)
Choosing the correct NFt format is a structured process that ensures your label meets Canadian regulatory requirements while remaining clear and practical for consumers. The steps below outline the core considerations every manufacturer should follow before finalizing an NFt for their product.
Step 1: Determine the Available Display Surface (ADS)

Begin by calculating the ADS of your package. This includes all surfaces where a label can reasonably appear, excluding areas such as the UPC, surfaces destroyed upon opening, areas that cannot be labelled, and bottoms of containers that would leak or be damaged if inverted. The ADS determines the allowable NFt size and whether space-saving formats are permitted.
Step 2: Identify Whether the Product Is Single-Serving or Multiple-Serving

The serving size rules and eligible NFt formats depend on whether the product is consumed in one sitting or over multiple occasions. Single-serving products can use specialized simplified formats, while multiple-serving foods require formats that pair household measures with metric equivalents.
Step 3: Confirm the Applicable Reference Amount (RA)

RAs are regulated quantities that reflect how much of a food is typically consumed at one time. Identifying the correct RA ensures your serving size aligns with Health Canada’s expectations, supports proper %DV calculations, and determines whether a product qualifies as single- or multiple-serving.
Step 4: Select the Appropriate NFt Format Family

Review the format families outlined in the FDR to determine which category fits your product. Standard formats work for most foods. Simplified formats apply to products with many zero-value nutrients. Dual formats apply to foods that require preparation or are customarily eaten with added ingredients. Aggregate formats are used when a product contains distinct components that need separate nutrient disclosures. Choosing the correct family ensures your declaration reflects how consumers will use the food.
Step 5: Choose the Correct Template Size From the Directory

Once the format family is selected, choose the specific NFt template and size from the Directory of Nutrition Facts Table Formats. The table must be legible, proportionate to the package, and as close as reasonably possible to occupying 15% of the ADS. If the largest permitted format is smaller than 15% of the ADS, manufacturers may still use it without enlarging the NFt beyond the prescribed maximum dimensions.
Final Remarks
Navigating Canada’s Nutrition Facts table requirements involves far more than simply placing nutritional data on a label. It requires a clear understanding of how serving sizes, reference amounts, Daily Values, and format families interact within a tightly regulated framework. Each decision, from calculating the available display surface to selecting the correct format family, contributes to the accuracy, clarity, and regulatory integrity of the final label. When applied properly, these requirements help ensure that consumers receive consistent information presented in a manner that is easy to understand, compare, and apply to their daily food choices.
For manufacturers and importers, the most effective approach is one that prioritizes accuracy from the outset and integrates compliance considerations throughout the product development and label design process. This reduces the risk of costly redesigns, market delays, or non-compliance findings during inspection. Although the regulatory landscape can appear complex, a structured and informed approach ensures that every element of the Nutrition Facts table aligns with the expectations of Health Canada and the CFIA.

To learn how Source Nutraceutical Inc. can support your team with regulatory planning, product reformulation, or cross-border labelling compliance, contact us today. Our experts are here to help ensure your food products remain competitive, compliant, and market ready.
🥐 More about our services here.
💡 Compliance is easy with the right support!
📩 info@sourcenutra.com
⬇️ Send us a request for support or an introductory call
FAQ
What information must appear in the Nutrition Facts table in Canada?
The NFt must include serving size, calories, fat, saturated and trans fats, carbohydrates, fibre, sugars, protein, cholesterol, sodium, potassium, calcium, iron, and the percent Daily Value for each. All content must follow the formats and rules outlined in the FDR.
How do I determine the correct serving size for the NFt?
Serving sizes must follow Health Canada’s regulated Reference Amounts. They generally reflect the food as sold, unless the food is typically consumed only after preparation, such as coffee, tea, or dry mixes.
Which foods are exempt from showing a Nutrition Facts table?
Some foods, such as alcoholic beverages, fresh fruits and vegetables without added ingredients, and certain small-package items, qualify for exemptions. However, exemptions may be lost if quantitative nutrient statements are added to the label.
How do I choose the right NFt format or size for my product?
Manufacturers must calculate the available display surface (ADS) of the package and select an NFt from the format family permitted for that product type. The chosen format must be proportionate to the ADS and comply with the layout and size requirements in the FDR.
Can I add nutrient or ingredient information outside the Nutrition Facts table?
Yes, quantitative statements can appear elsewhere on the label if they use the correct units and wording. However, adding such statements may trigger additional mandatory declarations within the NFt, depending on the nutrient.
✷ The content on this website, including information presented in this post, is provided for general informational purposes only and does not constitute legal, regulatory, or professional advice. While efforts are made to ensure accuracy, laws and regulations vary by jurisdiction and may change over time. Readers should not rely on this information as a substitute for advice from qualified legal or regulatory professionals. We disclaim any liability for actions taken based on this content, and users are encouraged to seek guidance specific to their circumstances.