Natural and Non-prescription Health Products Directorate Performance Standards
What Does This Mean For Your Product?
On March 23, 2022, the Natural and Non-prescription Health Products Directorate (NNHPD) of Health Canada held a meeting on Performance standards for NHP applications with NHP industry associations.
This meeting summarizes the current performance standards, some tips for better applications, and a glimpse into the service standards and refusal rates from the last 10 months.
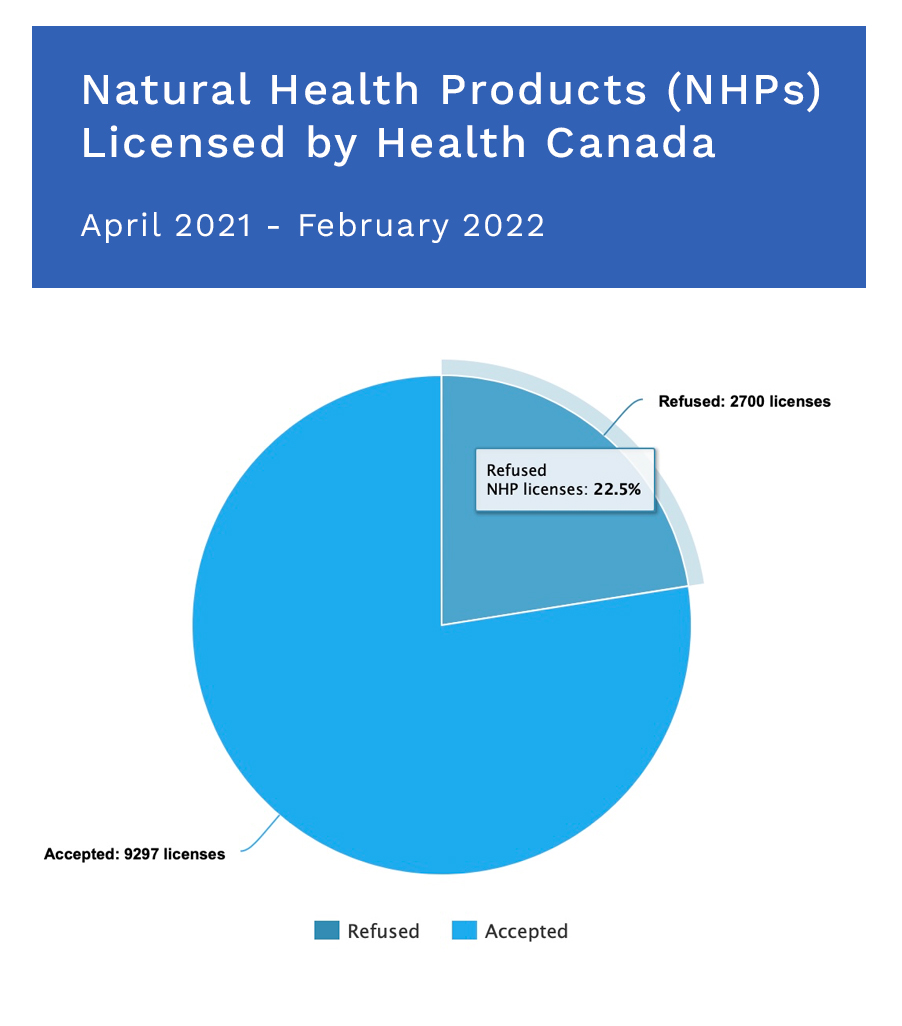
In the period of April 2021 to February 2022, Health Canada licensed 9,297 Natural Health Products (NHPs). Of that, 53% were Class 1, 13% were Class 2 and 34% were Class 3.
What’s not noted above is the refusal of 2,700 of those product license submissions.
For Site License submissions in that time period, 718 sites were licensed or renewed, and 377 submissions were refused. Refusals can be issued for a number of reasons when an application does not meet the requirements outlined in the regulations.
Here are just a few of the reasons why an application could be issued a refusal:
- missing contact information or Designated Party Authorization (DPA) form;
- incorrect or incomplete application form(s);
- application submitted in an unacceptable format;
- application contains damaged/corrupted file(s);
- failure to provide minimum application requirements including supporting evidence for safety and efficacy.
- failure to meet the parameter(s) of an NNHPD monograph to which the product attested.
- failure to submit the requested information in response to an Information Request Notice (IRN)




