In Case you Missed it: BioEnterprise Webinar - Your Pathway to the Canadian Market
Earlier this month, in partnership with Bioenterprise Canada, SNI’s very own Leanne Lutomsky and Marcel Fisette presented a webinar on regulatory topics such as packaging, health claims, ingredients, common food violations, and more. Bioenterprise Canada is network of entrepreneurs, accelerators, and service partners, committed to driving innovation across Canada in the agri-tech and food industry.

Below are some of the highlights discussed regarding the Canadian market:
- Common food violations include the use of non-permitted ingredients, non-permitted addition of vitamins and minerals, and non-permitted claims such as drug claims, misleading claims, and unsubstantiated claims.
- When determining the proper pathway for your product, it is important to consider composition, representation, format and history of use.
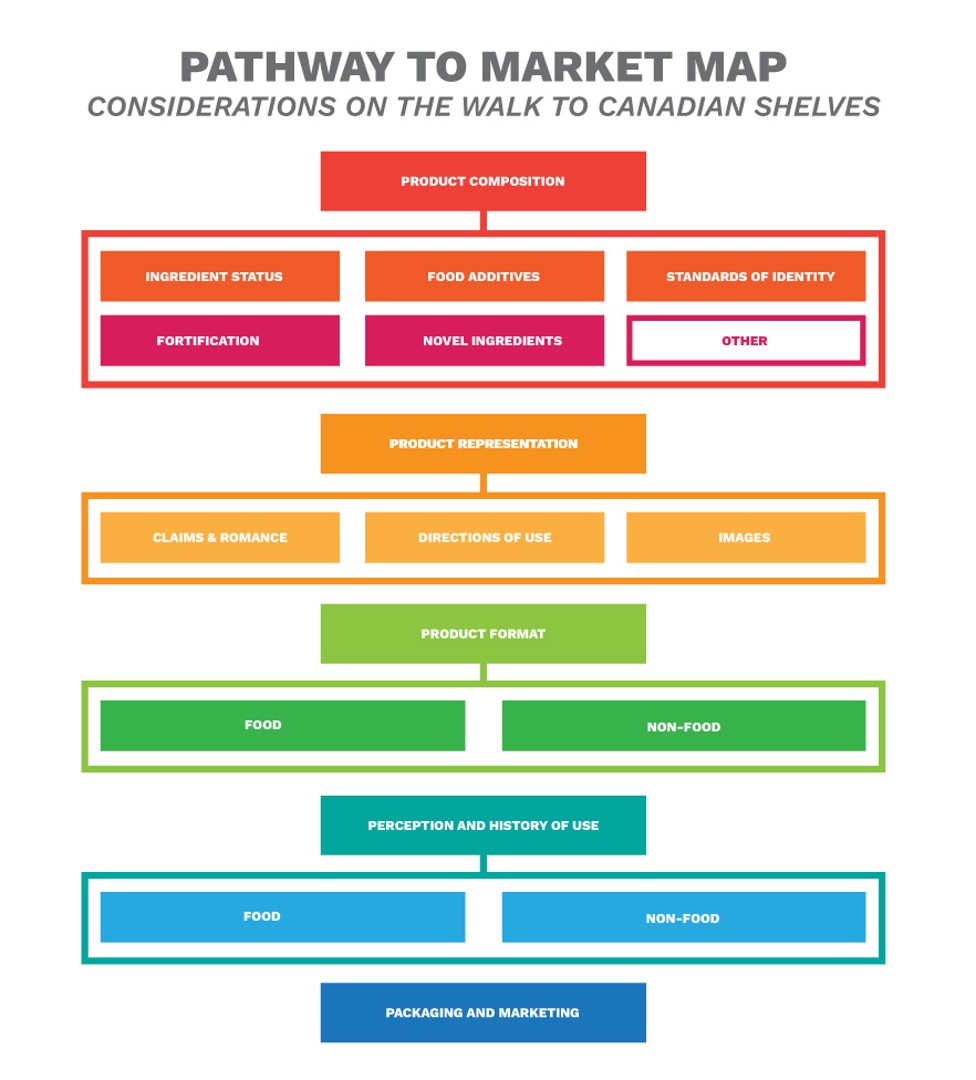
- You cannot simply add vitamins or minerals to any generic food product. There are specific lists of food which are permitted to be fortified, but if your product is not on the list there are a couple options. A Temporary Market Authorization can permit the sale of products that do not comply with the regulations on a case-by-case basis and under specific conditions. The proposed Supplemented Foods regulations are expected to come into force later this year, creating a new pathway for food products which are supplemented.
- A standard of identity (or compositional standard) sets out what ingredients a product must contain, what ingredients it may contain, and any requirements of manufacturing. Only if the ingredient meets the standard can it use the standardized name. There are approximately 300 standardized ingredients including ingredients like chocolate, flour, milk, soybean oil, and flavourings.
- Selling your product in Canada requires not only that your package be fully bilingual but also that a long list of packaging regulations be met, such as type height, claims, compliant Nutrition Facts tables and ingredient listings, etc. If these criteria is not met, it can trigger a labelling violation from CFIA. Depending on the severity of the violation, corrective action will be required. It can be as simple as updating artwork for the next production run, to as severe as a product recall. Recalls can be a result of missed allergen statements or incorrect ingredient declarations.




