When foreign facilities manufacture, package, or label Natural Health Products (NHPs) for the Canadian market, Health Canada requires proof that these sites meet Good Manufacturing Practices (GMPs). To simplify this process, the Natural and Non-prescription Health Products Directorate (NNHPD) issues a Foreign Site Reference Number (FSRN).
An FSRN acts as pre-cleared GMP evidence that can be referenced by Canadian importers in their site licence applications, reducing duplication and protecting confidential information. In this article, we explain what an FSRN is, who needs one, how the application process works, and the common pitfalls to avoid when applying.
What is a Foreign Site Reference Number (FSRN)?
A Foreign Site Reference Number (FSRN) is a unique identifier issued by the Natural and Non-prescription Health Products Directorate (NNHPD) of Health Canada to non-Canadian sites that manufacture, package, or label Natural Health Products (NHPs). It confirms that the foreign site has been assessed and found compliant with Part 3, Good Manufacturing Practices (GMP), of the Natural Health Products Regulations.
Why is a Foreign Site Reference Number (FSRN) Required?

Before NHPs can be imported into Canada, Canadian companies must hold a valid site licence that covers the activities they intend to perform, such as packaging, labelling, manufacturing, and importation. When NHPs are produced outside Canada, the Canadian site-licence holder must link the foreign site to their site licence. This process requires submission of GMP evidence to Health Canada, who reviews the information to confirm compliance of the foreign manufacturer. Once compliance is established, the foreign site can be linked, allowing importation to proceed.
To streamline this process, foreign manufacturers may apply directly to Health Canada for an FSRN. Applications are submitted through the WebSLA system and must be associated with an active Canadian site-licence application. The application outlines the foreign site’s activities, such as manufacturing, packaging, labelling, or warehousing, and provides GMP evidence to demonstrate compliance with the requirements of the Regulations.
Benefits of Obtaining a Foreign Site Reference Number (FSRN)
When granted, an FSRN serves as pre-cleared GMP evidence that Canadian importers can reference in their site licence submissions. This eliminates the need for each importer to submit duplicate documentation and helps protect confidential information belonging to the foreign site.
For foreign sites, an FSRN offers several advantages. It enables direct communication with Health Canada, safeguards sensitive information, and reduces administrative burden, particularly for sites working with multiple Canadian importers, since GMP evidence only needs to be submitted once.
An FSRN may be valid for one year or three years, depending on the type of GMP evidence provided. Because it is considered pre-cleared evidence, site licence applications that reference a valid FSRN are generally subject to shorter service standards. That said, product-specific information from the foreign site is often still required during the annexation process.
It is also important to note that an FSRN is not a site licence and does not authorize the direct export of NHPs into Canada. Foreign sites holding an FSRN must still partner with a licensed Canadian importer to supply products to the Canadian market.
Why is an FSRN Needed for Natural Health Products?
An FSRN confirms that Health Canada has reviewed and accepted a foreign site’s Good Manufacturing Practices (GMP) evidence for specific activities such as manufacturing, packaging, labelling, or warehousing. This confirmation carries significant value for both Canadian importers and foreign manufacturers.
For Canadian importers, an FSRN provides a streamlined path to compliance. Instead of compiling and resubmitting GMP documentation each time a foreign site is identified on a site licence application, the importer can reference the FSRN. This reduces administrative burden, expedites Health Canada’s review process, and minimizes the risk of regulatory delays that could impact product launches or supply chain continuity.

For foreign manufacturers, holding an FSRN positions itself as a reliable and compliant partner for the Canadian market. Importers are more likely to work with sites that already have an existing FSRN in place, as it allows them to move forward quickly and with confidence that Health Canada has already assessed the site’s GMP standards. This can provide a competitive advantage when establishing or expanding business relationships with Canadian companies.
From a regulatory perspective, the FSRN also strengthens oversight and accountability. Each identifier is linked to a specific foreign site and its approved activities, enabling Health Canada to maintain traceability and ensure that only compliant facilities are supplying NHPs to the Canadian market. In this way, the FSRN not only simplifies compliance processes but also contributes to the integrity and safety of the overall supply chain.
How to Apply for a Foreign Site Reference Number (FSRN)
Foreign sites that intend to supply Natural Health Products (NHPs) to the Canadian market may apply directly to Health Canada for a Foreign Site Reference Number (FSRN). Applications are submitted electronically through Health Canada’s online system. Linking a new FSRN submission to an active Canadian site licence helps ensure prioritization, resulting in a faster review compared to applications that are not associated with a site licence.
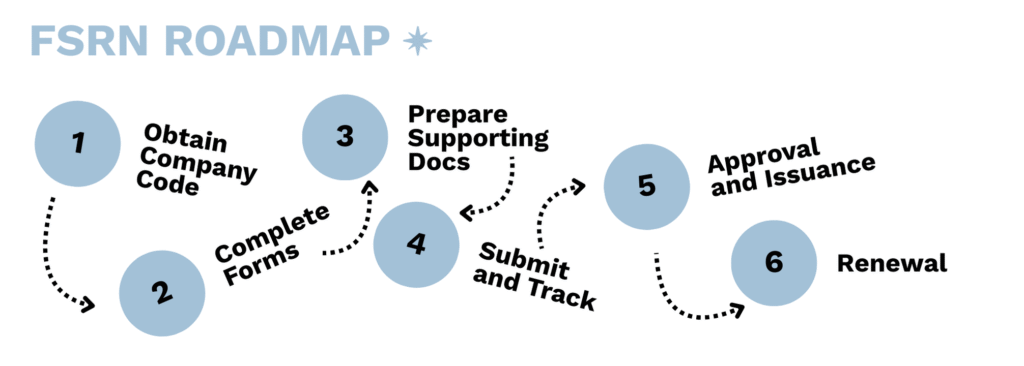
Step 1: Obtain a Company Code

Before starting the application, each company must first obtain a unique five-digit company code. This code identifies the company in Health Canada’s system and must be included in the application. To request a code, companies should email the Natural and Non-prescription Health Products Directorate (NNHPD) at nnhpd-dpsnso@hc-sc.gc.ca with their company details.
Step 2: Complete the Application Form

Foreign sites must complete the official application form (Foreign Site Reference Number (FSRN) Application Form), which allows them to apply for an FSRN and outline the activities performed at their facility. Activities can include manufacturing, packaging, labelling, or warehousing, and the application should clearly indicate the types of products handled, such as sterile, non-sterile, or homeopathic.
Step 3: Prepare Supporting Documentation

Applicants must submit GMP evidence demonstrating compliance with Canadian requirements. This may include a Quality Assurance Report (QAR) signed by a qualified quality assurance professional or other recognized GMP certifications. The documentation should describe the site’s facilities, equipment, procedures, processes, training, and quality systems in detail. Records demonstrating how GMP compliance is achieved are also required (i.e. batch records, pest control records, standard operating procedures, etc.).

It is important to note that the NNHPD will only issue a Foreign Site Reference Number (FSRN) when the applicant provides a complete submission containing all of the required information. If the application is missing key elements, or if the GMP evidence does not meet the standards set out in the Natural Health Products Regulations, the request may be refused. For many foreign companies, partnering with a consulting firm that specializes in NHP compliance can be a practical investment. Expert support helps ensure that the application package is accurate, complete, and aligned with Health Canada’s requirements, which ultimately saves time, reduces costs, and minimizes the risk of delays.
Step 4: Submit and Track the Application

Once the application and supporting documents are complete, they must be submitted electronically to Health Canada through ePost Connect. To set up ePost Connect for submissions, companies can email NNHPD.skmd.so-dgps.dpsnso@hc-sc.gc.ca to request an invitation link. Applicants should be prepared to respond to requests for additional information to support their FSRN request in a time-sensitive manner.
Step 5: Approval and Issuance
If the submission meets Health Canada’s requirements, the site will be assigned a Foreign Site Reference Number (FSRN). This number confirms that Health Canada has reviewed and accepted the site’s GMP evidence. The FSRN can then be shared with Canadian importers, who may use it to support their own site licence applications without needing to resubmit the same GMP documentation.
Step 6: Renewal and Maintenance
An FSRN is not permanent and must be kept up to date. Validity depends on the type of GMP evidence provided, and renewal requires proof of ongoing compliance. Sites are expected to maintain their quality systems and provide updated documentation to support their renewal at the frequency determined by Health Canada.
FSRN Renewal, Amendments, and Notifications
A Foreign Site Reference Number (FSRN) is not permanent and must be kept accurate and current to remain valid.
FSRN Renewal Requirements
Renewing a Foreign Site Reference Number (FSRN) is an essential step in demonstrating that a site continues to comply with Good Manufacturing Practices (GMP). Renewal provides Health Canada with assurance that the facility remains in compliance with the Natural Health Products Regulations and that its quality systems are being maintained.
The validity period of an FSRN depends on the type of GMP evidence submitted with the original application. When a Quality Assurance Report (WAR) is used, the FSRN is typically valid for one year from issuance. When pre-cleared GMP evidence is provided, the FSRN is valid for three years.
FSRN renewal applications must be submitted at least 30 calendar days before the expiry date to prevent interruptions. Renewal packages should include updated inspection reports, revised quality system procedures, records, and evidence confirming that corrective actions have been completed where required. However, because current review timelines may exceed 30 days, it is strongly recommended to file renewal applications well in advance of this minimum to avoid disruptions. Once a renewal has been submitted and accepted by Health Canada for processing, the existing FSRN remains valid until the renewal is finalized.
Importantly, Health Canada does not issue reminders for FSRN renewal deadlines. It is the responsibility of the FSRN holder to track and manage their renewal cycle. By keeping renewals timely and complete, foreign sites can ensure their FSRN remains active and continue to support Canadian importers without delays.
FSRN Amendments for Operational Changes
Amendments are an essential part of keeping a Foreign Site Reference Number (FSRN) accurate and valid. They are required when significant changes occur at a foreign site, such as adding new activities like packaging or labelling, relocating to a new address, expanding into new dosage forms, or introducing sterile manufacturing processes. These types of changes alter the scope of the site’s operations and must be reflected in Health Canada’s records to ensure continued compliance with the Natural Health Products Regulations.

To process an amendment, the FSRN holder must submit an updated application through Health Canada’s ePost system, along with revised GMP evidence and supporting business documents that demonstrate the site’s updated activities. Canadian importers that reference the FSRN must also be notified so they can update their own site licences accordingly. By managing amendments proactively, foreign sites help avoid compliance gaps, prevent delays in importer submissions, and maintain uninterrupted market access in Canada
FSRN Notifications for Minor Updates
Not all updates to a Foreign Site Reference Number (FSRN) require a formal amendment. Minor changes, such as an update to a site’s legal name, new contact information, or small adjustments in equipment or processes, are generally handled through a notification rather than an amendment. This approach allows Health Canada to keep its records accurate without requiring a full reassessment of the site’s GMP evidence.
Notifications must be submitted within 60 calendar days of the change. The submission should include an updated WebSLA form and a clear explanation of the modification so that Health Canada can update its records efficiently.
By keeping renewals, amendments, and notifications up to date, companies ensure that their FSRN accurately reflects their “live” operation status. This helps maintain uninterrupted market access, reduces the risk of compliance issues, and builds confidence with Canadian partners who rely on a foreign site’s FSRN as proof of GMP compliance.
Using a Foreign Site Reference Number (FSRN)
Once a Foreign Site Reference Number (FSRN) has been issued by Health Canada, it can be used to support a Canadian importer’s site licence application. Every foreign site involved in the manufacture, packaging, labelling, or warehousing of Natural Health Products (NHPs) destined for Canada must be listed on the importer’s site licence.
Once again, it is important to remember that an FSRN is not a direct authorization for export of NHPs into Canada. Rather, it is a reference number that demonstrates GMP compliance for a specific foreign site. To bring products into Canada, the foreign site must partner with a licensed Canadian importer who holds a valid site licence that includes the foreign site.
Health Canada Processing Timelines
Health Canada has established service standards for processing site licence and Foreign Site Reference Number (FSRN) applications. The timelines are measured in business days and vary depending on the type of GMP evidence submitted and the number of sites involved.
- Administrative processing to verify application completeness: 5 days*
- Applications with pre-cleared GMP evidence for all sites (no limit on number of sites): 30 days*
- Applications with one to nine sites that include a Quality Assurance Report (QAR) as GMP evidence for one or more sites: 60 days*
* Although Health Canada aims to meet its published service standards, applicants should anticipate potential delays. The most up-to-date submission timelines are communicated to applicants when their application is accepted for review.
Common Pitfalls to Avoid in FSRN Applications

Although a Foreign Site Reference Number (FSRN) can streamline compliance, many applications are delayed or refused due to preventable errors. One of the most common issues is the submission of incomplete or outdated documentation. Expired audit reports, unsigned quality agreements, or incomplete standard operating procedures (SOPs) often trigger additional information requests or result in outright refusal by Health Canada.

Inconsistencies in site information are another frequent problem. All details, including legal names, addresses, and the scope of activities, must match exactly across every document. Even minor discrepancies can lead to screening failures. Health Canada also applies strict refusal criteria, which means that applications may be rejected if they are submitted on outdated forms, are missing cover letters or Designated Party Authorization (DPA) forms or lack essential good manufacturing practice (GMP) evidence.

A further area of concern is the submission of insufficient activity-specific GMP documentation and records. Sites that perform multiple activities, such as manufacturing, packaging, or labelling, must provide GMP evidence for each activity. Evidence that covers only one function is not acceptable. To avoid these pitfalls, applicants should ensure they are using current application forms, verify that all information is consistent, and provide comprehensive GMP documentation that reflects the full scope of operations.
Final Remarks
A Foreign Site Reference Number (FSRN) is an important tool for both Canadian importers and foreign manufacturers of Natural Health Products (NHPs). By serving as pre-cleared GMP evidence, it reduces duplication, protects confidential information, and streamlines Health Canada’s review process. For foreign sites, holding an FSRN demonstrates compliance with Canadian standards, positions them as preferred partners in the marketplace, and creates efficiencies when working with multiple Canadian importers.
Success with FSRN applications depends on preparation, accuracy, and timeliness. Complete documentation, consistent site information, and activity-specific GMP evidence are essential for avoiding delays and refusals. Staying on top of renewals, amendments, and notifications ensures that the FSRN remains current and reliable. With a clear understanding of the application steps, processing timelines, and common pitfalls, companies can better navigate Health Canada’s requirements and maintain uninterrupted access to the Canadian NHP market.

Need support with FSRNs or other Health Canada submissions?
At Source Nutraceutical Inc. (SNI), we help brands navigate the complexities of Canadian regulatory compliance with confidence. Our team has over 20 years of experience supporting companies in bringing natural health products, foods, cosmetics, medical devices, and pet products to market.
From foreign site reference number (FSRN) submissions to site licence applications, clinical trials, and compliant labelling, we provide tailored solutions that reduce risk and streamline approvals. Whether you are an ambitious start-up or a global brand, SNI delivers the regulatory expertise, clinical evidence, and creative services you need to launch, grow, and expand in Canada and beyond.
✔️ More about our services here.
💡 Compliance is easy with the right support!
📩 info@sourcenutra.com
⬇️ Send us a request for support or an introductory call
FAQ
What is an FSRN and how does it support Canadian importers?
A Foreign Site Reference Number (FSRN) is a unique identifier issued by Health Canada to foreign sites that manufacture, package, or label Natural Health Products (NHPs). It confirms that the site has demonstrated compliance with Canadian Good Manufacturing Practices (GMPs). Canadian importers can reference an FSRN in their site licence applications, which simplifies the process and reduces the need to resubmit the same GMP information.
How do I apply for a FSRN, and what documents are required?
Foreign sites must apply through Health Canada’s electronic submission system (ePost) and link the application to an active Canadian site licence. The application must include GMP evidence such as a Quality Assurance Report (QAR) or inspection certificates from recognized authorities, along with details about the site’s activities and facilities and records of evidence of complying with Canadian GMPs for NHPs.
What are the processing timelines for FSRN applications?
Health Canada (NNHPD) has published service standards for FSRN and site licence applications. Administrative completeness checks are conducted within ~5 business days. For applications that provide pre-cleared GMP evidence, decisions are targeted within 30 business days; when a Quality Assurance Report (QAR) is used (for 1–9 sites), the target is 60 business days (for 10+ sites, up to 90 business days may apply). If a submission is deficient or refused, the review is paused, and resubmission is treated as a new application.
These service standards are targets only, and actual timelines may vary depending on submission volume, prioritization, complexity, and backlog. As a result, establishing a firm estimate can be difficult. Applicants receive the most up-to-date timelines when their application is formally accepted for review.
How long is an FSRN valid, and how are renewals managed?
The validity of an FSRN depends on the type of GMP evidence submitted. An FSRN based on a Quality Assurance Report (QAR) is typically valid for one year, while one supported by pre-cleared GMP evidence is typically valid for three years. Renewals require updated inspection reports and documentation that confirm ongoing compliance. In some cases, Health Canada may extend validity if the site has been inspected recently and no significant risks were identified. Foreign sites must manage their own renewal timelines.
What are common pitfalls that can cause delays or refusals?
The most common mistakes include incomplete or outdated documentation, inconsistent site details across forms, and missing evidence for specific activities. Health Canada requires that all applications be complete, accurate, and submitted using current forms. Foreign sites that perform multiple activities, such as manufacturing, packaging, and labelling, must provide GMP evidence for each one. Avoiding these pitfalls helps ensure smoother reviews and reduces the risk of delays.
✷ The content on this website, including information presented in this post, is provided for general informational purposes only and does not constitute legal, regulatory, or professional advice. While efforts are made to ensure accuracy, laws and regulations vary by jurisdiction and may change over time. Readers should not rely on this information as a substitute for advice from qualified legal or regulatory professionals. We disclaim any liability for actions taken based on this content, and users are encouraged to seek guidance specific to their circumstances.




