Earlier this year we informed you about the upcoming Supplemented Food Regulations and what to expect as a food or natural health brand.
In this post, the first of a series, we delve deeper into the Supplemented Food Regulations to help you understand the nuances of this new category and what it means for brands selling in Canada.
Part I highlights new definitions, label requirements, and how to know if your product falls into the supplemented foods framework.
MAKING SENSE OF THE NEW FRAMEWORK,
AND HOW IT HELPS CONSUMERS
As times and trends evolve, we have seen new food innovations enter the market that were not anticipated when the Food and Drug Regulations were developed. This has led to a regulatory gap for these products.
Of particular concern is supplemented foods, where supplemental ingredients added to foods can pose a risk if overconsumed in the general population.
What Happened to Temporary Marketing Authorizations (TMAs)?
To address this issue, years ago Health Canada developed long-term plans to better handle how supplemented foods are regulated. The interim solution was to authorize supplemented foods through Temporary Marketing Authorization Letters (TMALs).
TMALs are no longer being issued, and previous TMALs are set to expire within the coming years. For certain products, a transition period ending December 31, 2025 was provided to give time to update labels. Other products which had TMALs are no longer compliant under the Supplemented Food Regulations and extensions beyond their TMAL expiry date may not be possible.
What Are Considered Supplemented Foods?
According to Health Canada’s definition, “Supplemented foods are prepackaged foods with added supplemental ingredients such as vitamins, minerals, amino acids and/or other ingredients (e.g., caffeine, herbal ingredients).”
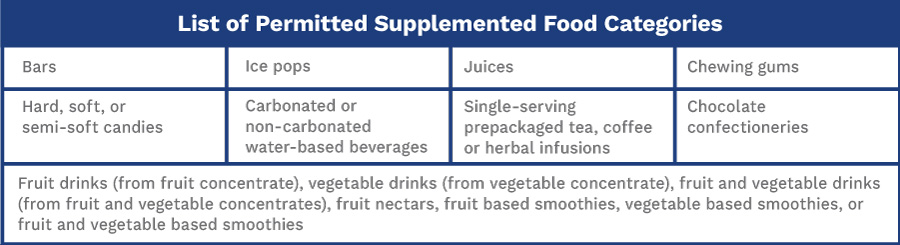
What Are Supplemental Ingredients?
Another new definition to know is supplemental ingredient. A supplemental ingredient is “a nutrient — including a vitamin, mineral nutrient or amino acid — or any other substance listed in column 1 of the List of Permitted Supplemental Ingredients and added as an ingredient to a food in accordance with the applicable conditions of use set out in columns 2 to 5.”
Other examples of supplemental ingredients include:
CAFFEINE
TAURINE
L-CYSTEINE
HERBAL INGREDIENTS
When a supplemented food is used as an ingredient in another supplemented food, the supplemental ingredients in the first food are also supplemental ingredients in the second food (not components).
It’s important to note that supplemental ingredients and supplemented foods cannot be used as ingredients in prepackaged products that are not supplemented foods. This would be considered as adulterated and therefore prohibited for sale.
What is NOT Considered a Supplemented Food?
Certain products are not applicable to the new regulations.
Supplemented foods do NOT include:
- Foods for special dietary use – except gluten free foods;
- Foods labelled and advertised for consumption by children under four years of age, pregnant or breastfeeding women;
- Foods that are fortified for public health purposes – except prepackaged water;
- Foods that have not been processed or have been minimally processed; and
- Beverages with an alcohol content of more than 0.5%
LABELLING REQUIREMENTS
FOR SUPPLEMENTED FOODS
Supplemented foods have their own unique labelling requirements that are different than any other product category.
The products still must comply with general labelling and advertising requirements in the Food and Drugs Act and Regulations, and Safe Food for Canadians Act and Regulations. However, in addition to these, supplemented foods also must satisfy the following unique requirements:
- Supplemented Food Facts table (SFFt)
- List of cautionary statements, if applicable
- Supplemented Food Caution Identifier (SFCI), if applicable
Does My Product Need a Cautionary Statement?
Depending on the level of supplemental ingredients contained in a product, some supplemented foods require cautionary statements and a caution identifier to be displayed on the label.
There are two main formats for the caution identifier: standard and compact. Determining which to use will depend on the principal display surface area, and the standard format will be required most often.
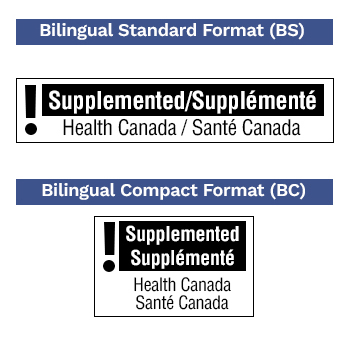
Criteria for including a cautionary statement are based on threshold levels for each individual vitamin, mineral, amino acid, and certain other ingredients as well as recommendations for caffeine.
Need help decoding this and determining what is applicable for your product?
How Can I Comply with the Supplemented Food Regulations?
If you need help meeting these new compliance requirements for supplemented foods, our experienced regulatory affairs team can help!
At SNI, we specialize in both regulatory consulting and creative services, including food label design and packaging compliance. We will help you stay compliant so you can keep your brand trusted and on the shelves.
>> Contact us today to discuss options for your brand.
Watch for the next post in the series where we’ll share tips on formulating supplemented foods that are compliant – and competitive.




