The new class of Cannabis Regulations have been finalized. These regulations will come into force on October 17, 2019 and new products will be available for sale by mid-December 2019 at the earliest to allow you to stuff your Christmas stockings or to ring in the New Year with whatever you may fancy-some edible cannabis, cannabis extracts or cannabis topicals.
Licence holders will need to inform Health Canada of their new cannabis products at least 60 days prior to sale. The notifications will only be accepted on October 17,2019. Health Canada encourages licence holders to build inventory of the new cannabis products they intend to sell and properly plan out their manufacturing.
No amendments have been proposed to Licensing, Test Kits, Reporting nor Personnel and Physical Security clauses of the Regulations. Licence holders conducting activities with the new classes of cannabis will be subject to the same strict physical and personnel security requirements established under the Regulations.
What’s changed? Noteworthy amendments to the Regulations include;
All in the name of Good Production Practices:

- Cleanliness of equipment now includes conveyances (such as a forklift) that handles cannabis or ingredients within the licenced facility.
- A natural or mechanical ventilation system providing clean air and removes unclean air is required in areas that may contaminate the cannabis or ingredients.
- Sanitation program explicitly requires hand cleaning and sanitizing stations and lavatories on a licensed site along with appropriate clothing and coverings in good condition, clean and in sanitary conditions-gloves, the ever-lovely hairnet, beard net, smock.
- A preventive control plan (PCP) identifying and addressing the potential hazards is mandatory along with documented evidence demonstrating the control measures are meeting their intended purpose. Please ensure the Qualified Assurance Person (QAP) has signed off on your PCP prior to implementation.
- Competent and qualified individuals are required to conduct activities involving edible cannabis.
- The QAP or designated is required to be proactive and conduct investigations of any suspected cannabis or ingredient that may present a risk of human health injury.
- Steps are to be taken to prevent animals from entering the building or any part where cannabis is being produced. Cats are not an allowed method to control pests, for anyone still debating this issue.
- Water using in processing or coming into contact with cannabis or any ingredient has to be potable and not present any contamination risk.
- Proper segregation and separation will be required for waste disposal, contaminates along with incompatible activities.
- Production, packaging, labelling, distribution, storage, sampling and testing standard operating procedures (SOPs) are required for cannabis and their ingredients conducted in the licenced premise.
The sky’s the limit? Not quite:
- To reduce the overconsumption or accidental consumption, Health Canada has set limits as follows:
- 10 milligrams of THC per discrete unit and per immediate container
- If the label displays THC or CBD quantity exceeding 5 mg, the product has 15% variability limit
- If the label displays a THC or CBD quantity more than 2 mg but less than 5 mg, the variability limit is 20%
- The variability limit is 25% for a THC or CBD quantity less than 2 mg
An informative label:
As mentioned in our Regulating Weed Eater Blogpost, the nutritional information on edible cannabis is required to include:
- List of ingredients
- Common name of the cannabis product
- Allergen declaration (incl. sulfites) or part of ingredient listing
- Durable life date (best-before-date) with a durable life of 90 days or less

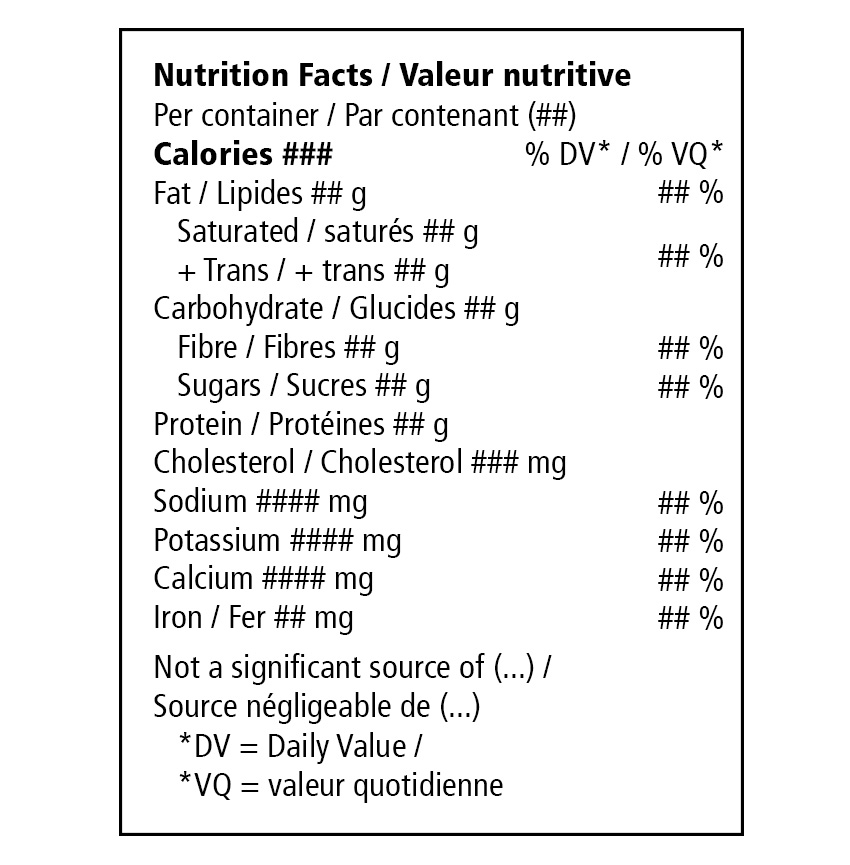
- Cannabis-specific nutrition facts table (NFT) – see image on the left
- Font size, font type, border, spacing all consistent with other labelling requirements
- 12 core nutrients, energy value (calories) and % DV as applicable to a “Per container/Par contenant” basis
- Health claims that would promote edible cannabis to meet particular dietary requirements or representing health benefits regardless if currently allowed on permitted food are prohibited
- The edible cannabis packaging is required to list the company name, email address of the processor that manufactured the product
- The package or label may not display testimonials, endorsements, depictions of a person, character or animal real or fictional or associate the product or the brand element with a particular lifestyle
- The cannabis health warning messages, standardized cannabis symbol and information in regard to THC and CBD content is required on the exterior display surface
Example:
WARNING: It can take up to 4 hours to feel the full effects from eating or drinking cannabis. Consuming more within this time period can result in adverse effects that may require medical attention.
Contact Source today to assist with your labeling needs and for any clarifications on the new regulations. We are here to help!





