SNI Clinical Research
SNI – July 4, 2022
As a full-service Canadian contract research organization, SNI has a wealth of experience supporting our clients along the research and development pathway: from the start of a product concept, to attaining a health claim on a marketed product! Why participate in a Clinical Trial? As a clinical trial participant,...
Read More
BULLETIN: FOP Labelling
SNI – June 30, 2022
THIS JUST IN! Front-of-Package Nutrition Labelling, as part of Health Canada’s Healthy Eating Strategy, will be mandatory as of January 1, 2026 and the symbol is now finalized. The regulations with all details are set to be published in the Canada Gazette, Part II on July 20, 2022. The purpose of...
Read More
NNHPD Consultation – Performance Standards
SNI – May 13, 2022
Natural and Non-prescription Health Products Directorate Performance Standards What Does This Mean For Your Product? On March 23, 2022, the Natural and Non-prescription Health Products Directorate (NNHPD) of Health Canada held a meeting on Performance standards for NHP applications with NHP industry associations. This meeting summarizes the current performance standards,...
Read More
Supplemented Food Regulations
SNI – May 2, 2022
There is a new pathway for your unique product! A regulatory framework to allow market access for supplemented foods without pre-market review and approval by Health Canada. Forget the Temporary Marketing Authorization! With the guidance of SNI’s regulatory team, your supplemented food product can be on Canadian shelves without further...
Read More
INSIDE SNI: Meet Maira and Madyson
SNI – March 3, 2022
As part of our efforts to introduce you to the faces behind SNI, we're announcing another issue of INSIDE SOURCE series and excited to exhibit two more members of our amazing team! Maira Medellin-Peña is our Clinical Trials and Regulatory Compliance Specialist. Maira comes to SNI with a diverse background...
Read More
Are your NFts Ready?
SNI – February 15, 2022
In 2016, the Canadian Food Inspection Agency (CFIA) announced new food labelling regulations. Recognizing the industry would need time to pivot, the CFIA allowed for a five-year transition phase, ending December 2021. As a result of additional challenges, an extra year was given before enforcement action would be applied, but...
Read More
Your Pictures Speak Volumes
SNI – January 28, 2022
We can transform your flat artwork into this... this... or even this. Our photo-realistic 3D renderings are perfect for planograms, sell sheets and much more. CONTACT US TO LEARN MORE.
Read More
UNFI Fall Vendor Summit: Recap
SNI – November 24, 2021
To help the community gain greater access to relevant resources and tools, United Natural Foods Inc. (UNFI) Canada hosted a vendor summit to bring all partners together to meet, touch base, and develop meaningful relationships. On November 17, 2021: Director of Business Development Stuart Greenfield and Creative Director Marcel Fisette were...
Read More
SNI Speaker with Venturepark Labs: Packaging Strategies
SNI – November 23, 2021
Venturepark Labs is a community of business, brand, culture, and CPG specialists. They can help all aspects of business—from funding some companies, to marketing others. They can help with scaling, reinventing, and strategizing to drive business growth. They are a true Innovation Hub. Here at SNI, we pride ourselves in the firm...
Read More
Plain Language Labelling Requirements in Canada: Natural Health Products
SNI – November 22, 2021
Health Canada is proposing amendments to the Natural Health Product Regulations which would introduce some key features in order to make labelling of NHPs more clear, consistent, and legible to the general public. This update would align with the newly implemented Plain Language labelling requirements for non-prescription drugs. With the...
Read More
Introducing Dr. Lindsei Sarna
SNI – November 16, 2021
Lindsei Sarna is SNI’s new Associate Director of Regulatory Affairs and Quality Assurance. Lindsei has a long-standing interest in evidence-based health products, and brings with her a strong and diverse technical background in the areas of biomedical research, natural health products, nutrition, cosmetics and cannabinoid research. Lindsei obtained a BSc...
Read More
The FDA is urging food manufacturers to reduce salt in products
SNI – October 20, 2021
In 2012, Health Canada published voluntary targets for reducing sodium in processed food by the end of 2016. The targets were developed through consultation with the food industry, health sector and research experts. Now, in an announcement made on Wednesday, the Food and Drug Administration is urging both restaurants and...
Read More
SNI New Research Clinic Opens this Fall
SNI – September 24, 2021
SNI is thrilled to announce the expansion of our capabilities as a full-service contract research organization to include an in-house clinical trials facility in Winnipeg (Manitoba, Canada). As we continue to offer expert strategic consulting services to help guide clients through Canadian and U.S. pre-market commercialization processes, we can now...
Read More
Source Nutraceutical, Inc. Has an Updated Look!
SNI – September 24, 2021
We’re excited to announce that Source Nutraceutical, Inc. has undergone a rebrand! Here at SNI, we’re thrilled to enter this new and exciting phase with a fresh and updated look that accurately reflects our growth as a company, and who we are today! Our New Logo After 18 years of...
Read More
Participate in a Research Trial
SNI – September 24, 2021
Participating in a research trial can be a rewarding experience. By giving your time as a research participant, you are helping researchers to develop and advance scientific discoveries. Depending on the clinical trial, participants can receive a copy of their individual results from the study, usually in the form of...
Read More
Take the Quiz: Are Your Labels Compliant?
SNI – September 17, 2021
Canadian Food Labelling Challenge – Take our quiz! Are your Labels in Compliance with Canada’s Food Labelling Requirements? QUESTION 1: THE NET QUANTITY OF A PREPACKAGED FOOD PRODUCT IS 355.2 GRAMS. WHAT IS THE PROPER BILINGUAL LABEL DECLARATION? 355.2 g 350 g 355 g 0.35 kg QUESTION 2: WHICH OF...
Read More
Save the Date… Canada Organic Week is Next Week!
SNI – September 9, 2021
What is Organic Week? Canada’s National Organic Week is the largest annual celebration of organic food, farming and products across the country. Annually, there are hundreds of individual events showcase the benefits of organic agriculture and its positive impact on the environment during this time. Organics represent a vibrant alternative...
Read More
Decentralized Clinical Trials during COVID-19
SNI – July 27, 2021
Earlier this year we talked about the challenges of conducting clinical research during COVID-19. The COVID-19 pandemic has brought us to a “New Normal” situation in clinical research, and we (as a contract research organization) have adapted to accommodate current health guidelines. Over the last 17 months we have seen...
Read More
The Future of Meat is…. Lab-Grown?
SNI – July 21, 2021
Lab-grown meat (also known as clean meat, cultured meat, cellular agriculture, or in-vitro meat) is simply flesh that is grown outside of an animal’s body. The meat itself is real, but it is obtained without slaughter. Aiming to disrupt the conventional ways of producing animal products, the goal of lab-grown...
Read More
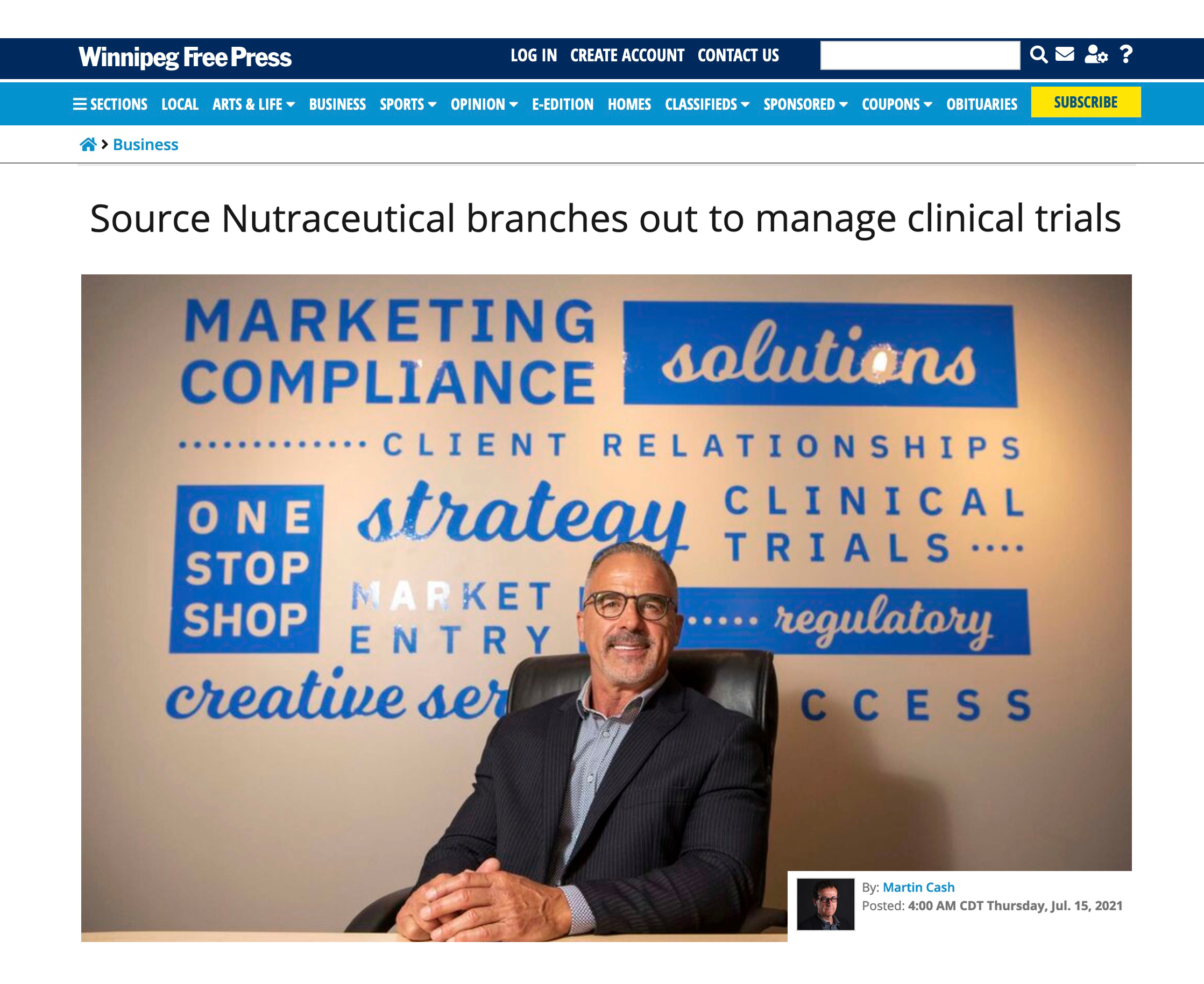
SNI Clinical Trial Services featured in Winnipeg Free Press
SNI – July 15, 2021
Our talented and fantastic team was featured in Winnipeg Free Press this morning–including a great photo of our President & CEO Bernie Desgagnés and a brief history of how far he has come leading us towards success. We’re so excited about managing clinical trial services and partnering up with the innovative team...
Read More